Pathogenisis Of Acne
/A brief review of the pathogenesis of acne will create the context for a discussion of the features of the disease in adults and the treatment approaches that target specific pathogenic factors.
The pilosebaceous unit (the sebaceous follicle,
sebaceous glands, and sebaceous ducts) is the site of acne.
Pilosebaceous units are concentrated in body sites that are prone to
acne
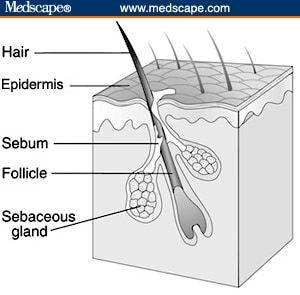
Figure 1. The pilosebaceous unit. (Courtesy NIH)
Excessive sebum production secondary to androgen stimulation.
The differentiation of the sebaceous epithelial cell (the sebocyte) and
the production of sebum are mainly under the control of androgen
hormones. During the prepubertal period, the increase in
adrenal androgens triggers the enlargement of the sebaceous glands,
with a consequent increase in sebum production. With the onset of
puberty and the increase in androgen from the gonads, sebaceous glands
become even more active. In acne patients, the number of
lobules in the sebaceous gland and the size of the sebaceous follicle
are increased compared with people without acne, as is the amount of sebum produced.
However, the components of sebum, which include squalene, wax esters,
triglycerides, cholesterol, and cholesterol esters, do not differ
between people with and without the disease. Excess sebum
production in people with acne is likely caused by differences in
end-organ (pilosebaceous unit) responsiveness to androgens and,
possibly, by increased circulating androgens.
Altered follicular keratinization and desquamation, resulting in follicular plugging. Sebum flows through the canal of the sebaceous follicle, which is lined with a keratinizing epithelium. In acne patients, there is increased production of the corneocytes lining the follicle and retention of these corneocytes within the follicle. The abnormally desquamated corneocytes and the excess sebum build up within the follicle to form a microscopic, bulging mass called the microcomedo. The trigger for corneocyte hyperproliferation is unknown, but androgen stimulation, decreased levels of follicular linoleic acid, increased levels of DHEAS, and interleukin (IL)-1 alpha activity have all been proposed as possible mechanisms.[6]
Proliferation of Propionibacterium acnes, an anaerobic organism normally resident in the follicle. The enclosed, sebum-rich environment of the sebaceous follicle is ideal for the proliferation of P acnes,
the anaerobic bacterium that produces chemotactic factors and recruits
proinflammatory molecules involved in the inflammatory phase of acne. P acnes counts have been shown to be higher in young people (age 16-21) with acne compared with nonacne controls, but no difference in P acnes counts was seen in people aged 21-25 with and without acne.
No differences are apparent in the cutaneous and follicular microflora
of adolescent acne patients, persistent acne patients, and late-onset
acne patients. There is no correlation between acne severity and skin-surface P acnes colonization, but even so, agents that reduce P acnes counts are clinically effective in the treatment of acne.
Inflammation following chemotaxis and the release of proinflammatory mediators. The proliferation of P acnes results in a variety of proinflammatory stimuli, including cytokines such as IL-1, IL-8, IL-12, and tumor necrosis factor-alpha, and proinflammatory byproducts including proteases, lipases, and chemotactic factors for neutrophils, lymphocytes, and macrophages. Follicular rupture and leakage of lipids, corneocytes, and bacteria into the dermis further exacerbate inflammation.
There
is also evidence, primarily from twin studies, that acne may be
inherited. One study of 204 patients over the age of 25 found that
familial factors were important in determining individual
susceptibility to adult persistent facial acne. The risk
of adult acne occurring in a relative of a patient with adult acne was
nearly 4 times greater than for a relative of an unaffected individual.





