Reloxin vs Botox
/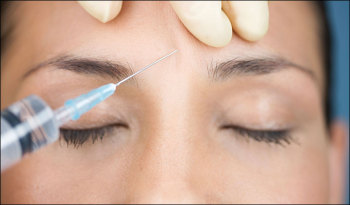 Reuters has this press release from French drug maker Ipsen about it's Botox killer, Reloxin:
Reuters has this press release from French drug maker Ipsen about it's Botox killer, Reloxin:
French drug maker Ipsen's (IPN.PA) anti-wrinkle treatment Reloxin, a possible rival to Allergan's (AGN.N) Botox, reduced forehead wrinkles and had few side effects in a U.S. study published on Monday.
Ipsen said in January the U.S. Food and Drug Administration had extended to April 13 its review of the company's injectable botulinum toxin product for possible approval.
A study involving 1,052 people, published in the Archives of Facial Plastic Surgery, showed that Reloxin improved the appearance of moderate to severe forehead lines.
Within a week, 93 percent to 95 percent of the people in the study responded to the treatment, consisting of five injections at a time, the researchers said. One treatment typically worked for almost three months.
The patients received up to five sets of five injections over the 13 months of the study.
Only one person dropped out of the study due to side effects related to the drug, the researchers said.
Ipsen in 2006 granted Scottsdale, Arizona-based Medicis Pharmaceutical Corp (MRX.N) rights to develop, distribute and commercialize Reloxin in the United States, Canada and Japan for aesthetic use and Medicis funded the study.
The company declined to comment on the findings, citing the ongoing FDA review. The drug is approved for fighting wrinkles in 23 countries, but not the lucrative U.S. market.
"It had a good safety profile and was effective, but we did not do a head-to-head comparison (with Botox). That's the next step, of course, something that probably will be done in the future," said Dr. Ronald Moy of the California Health & Longevity Institute, one of the researchers.
"It will be a good alternative for consumers and may be less expensive," Moy added in a telephone interview.
The drug is made from a toxin made by the bacterium Clostridium botulinum, which also causes botulism food poisoning.





