Expression As An Filler Injection? FDA Warning & Company Recall.
/Enhancement Medical recieved a warning letter from the FDA that takes them to task for allowing their product to be used as a "filler injection" which, according to the FDA, it was never approved for. In response, Enhancement Medical has recalled all lots of Expression.
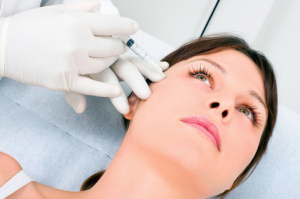 Photo: Wikipedia“The FDA has become aware of adverse events associated with the unapproved use of the Expression product as a dermal filler,” reads the warning. “The FDA has not approved this product for use as a dermal filler and recommends that health care providers stop using Expression by Enhancement Medical LLC as a subcutaneously administered substance.”
Photo: Wikipedia“The FDA has become aware of adverse events associated with the unapproved use of the Expression product as a dermal filler,” reads the warning. “The FDA has not approved this product for use as a dermal filler and recommends that health care providers stop using Expression by Enhancement Medical LLC as a subcutaneously administered substance.”
Manufactured by Enhancement Medical, Expression was approved by the FDA in 2012 as an intra-nasal splint to be used after nasal surgery. According to Enhancement Medical, it’s a "third-generation hyaluronic acid gel" that’s sulfite- and pathogen-free.
Unlike Juvederm, Restylane, Perlane, and other commonly used hyaluronic acid products, which are FDA-approved for cosmetic use, Expression is being used off-label. (Off-label use is common and not illegal. Botox was used for years off-label to treat wrinkles, migranes and sweating for which it had not yet been approved.) Nevertheless, in the past couple of years Expression has come into wider use by doctors and cosmetic clinics. The number of growing treatments has resulted in a correstponding increase in complaints.
In Feburary 2014 the FDA first contacted Enhancement Medical regarding complaints of adverse reactions and in June sent them a warning letter criticizing them for what the FDA perceived as inadiquate follow-up and investigation as well as using the term "filler injection" in the copy on their web site.
The FDA says that there are adverse events associated with the unapproved use of the Expression product, hyaluronic acid that is packaged in a syringe, as a dermal filler. Events have included swelling, tenderness, firmness, lumps, bumps, bruising, pain, redness, discoloration, itching, and the development of hard nodules.
Expression is listed with the FDA as an intranasal splint, and is intended to minimize bleeding and swelling and to prevent adhesions (sticking together) between the septum and the nasal cavity. Intranasal splints are placed in the nasal cavity after surgery or trauma and are usually constructed from plastic, silicone, or absorbent material.
The FDA issued a warning letter to Enhancement Medical LLC on June 4, 2014, advising the company of multiple quality system, correction/removal, and medical device reporting violations that were revealed during an inspection.From the FDA letter:
FDA also reviewed your firm’s medical device registration and listing, which lists the Expression as a Class I exempt intranasal splint. Generic devices classified under 21 CFR 874.4780 (Intranasal Splint, Class I) are intended to minimize bleeding and edema and to prevent adhesions between the septum and the nasal cavity. Intranasal splints are placed in the nasal cavity after surgery or trauma.
We note, however, that FDA is aware of and has reviewed multiple documents for Expression that contain claims, phrases and/or statements that refer to your firm’s device as an “injectable filler.”
For example, during FDA’s recent inspection, your firm provided the Investigator with a document entitled “The Science Behind Expression” which contained the following statements:
- “Enhancement Medical is using the new pathogen-free, raw HA material to develop and manufacture Expression™ injectable filler in Wauwatosa, Wisconsin.”
- “Enhancement Medical uses the raw HA product from Novozymes as a non-toxic and non-pathogen delivery medium for its Expression™ injectable filler.”
- “In formulating Expression™, the HA molecules are cross-linked with divinyl sulfone (DVS) and the gel is swelled to equilibrium. This provides the Expression injectable filler product with an 80/20 gel to fluid ration with up to 26 mg/mL of HA. Thus the formulation combined with the highly robust HA molecules result[s] in a very rich and potent filler compared to others.”
- “Clinicians find Expression™ does not have the hydrophilic effect when placed into tissue, eliminating guesswork from corrections and providing greater utility for multi-purpose use.”
As stated above, under 21 CFR 874.4780, intranasal splints are placed into the nasal cavity after surgery or trauma. Intranasal splints are not intended for injection through intact skin or mucosa and placement into tissue. Injection through intact skin or mucosa and placement into tissue may raise new questions of safety and effectiveness that could require either premarket approval and/or clearance before the device may be marketed in the United States. Please provide us with the basis for your identifying Expression as an “injectable filler” and whether you intend to continue making such claims. See 21 CFR 874.9(a) (limitations on exemption – promoting your device as an injectable filler may indicate a different use which could cause it to no longer be 510(k) exempt).
Additionally, FDA has noted that your firm’s website (www.enhancementmedical.com) contains the following statement for the Expression injectable filler: “Expression has FDA indication for use as intranasal splint.” Please be advised that this statement may be confusing to users and could create an impression of official approval of your device. As such, we request that your firm please remove any reference that FDA has indicated your device, Expression, for a specific use.
The response from Enhancement has been to recall all lots of Expression.
According to the recall letter the company received 99 complaints from 14,835 syringes shipped with compaints that included "swelling, redness, pain, bumps, firmness, bruising, itching, inflammatory reactions, infections and abscesses".
The letter continues, "We do not recommend that Expression be injected subcu-taneously for any reason because Expression’s safety and effectiveness as a subcutaneously administered substance has not been established in controlled clinical studies."
What's interesting here is that the list of complaints are pretty much exactly what you'd expect with any filler like Juvederm or Restylane. I'm wasn't able to find anything online that would call out Expression as having reactions that are abnormally high (althought they might be). If anyone has relevant stats please leave a comment below.
In any event, you'll want to send back your recalled Expression if you've been using it.





