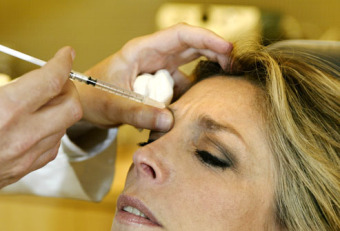
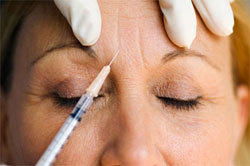
 Botox & Dysport now have a new contender in the cosmetic space... Xeomin.
Botox & Dysport now have a new contender in the cosmetic space... Xeomin.
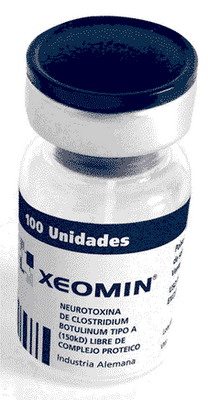
About Xeomin: (pronouced ZEE-oh-min) from Merz Pharma GmbH & Co KGaA
Download the Xeomin Report PDF
Botox, Dysport and Xeomin have a lot in common, but they also have some important differences. Unlike both Botox and Dysport, Xeomin does not need to be refrigerated before it's reconstituted (see below). This should be an advantage during distribution. What's more, Xeomin has no additives — just botulinum toxin type A. This may lessen a patient's likelihood of developing antibodies.
Supposedly, Xeomin is more like Botox than Dysport. It takes about one week for the full effects of Xeomin injections to be realized, and once this occurs the results last from three to six months. Dysport, Xeomin and Botox should not be used interchangeably.
Also, since Xeomin is approved only for cervical dystonia and blepharospasm in adults who have had previous treatments with onabotulinumtoxinA (Botox), any use for wrinkles and crows feet is going to be off label. This, along with the fact that Botox pretty much owns this space will probably mean that Xeomin will have a hard slog finding a huge audience. It may be worth trying thought to see if you just like it that much better. (Anyone who's already tried it, please leave a comment and let us know what you think.)
Storage
Unopened vials of XEOMIN® (incobotulinumtoxinA) can be stored at room temperature 20 to 25°C (68 to 77° F), in a refrigerator at 2 to 8°C (36 to 46°F), or a freezer at -20 to -10°C ( 4 to 14°F) for up to 36 months. Do not use after the expiration date on the vial. Reconstituted XEOMIN® (incobotulinumtoxinA) should be stored in a refrigerator at 2 to 8°C (36 to 46°F) and administered within 24 hours.
Indications & Usage
Cervical Dystonia: XEOMIN (incobotulinumtoxinA) is indicated for the treatment of adults with cervical dystonia to decrease the severity of abnormal head position and neck pain in both botulinum toxin-naïve and previously treated patients.
Blepharospasm: XEOMIN (incobotulinumtoxinA) is indicated for the treatment of adults with blepharospasm who were previously treated with onabotulinumtoxinA (Botox).
Complications
Like other botulinum products, Xeomin must carry a black box warning regarding a rare risk for spreading outside of the injection site. If this occurs, life-threatening swallowing and breathing problems may result. This has not been seen in people receiving neurotoxins for cosmetic reasons or to treat blepharospams. It has mainly occurred among children treated off-label for cerebral palsy-related muscle spasms.
Adverse Reactions
Cervical Dystonia: The most commonly observed adverse reactions (incidence ≥10% of patients and twice the rate of placebo) for XEOMIN 120 Units and XEOMIN 240 Units, respectively, were: dysphagia (13%, 18%), neck pain (7%, 15%), muscle weakness (7%, 11%), and musculoskeletal pain (7%, 4%).
Blepharospasm: The most common adverse reactions (incidence ≥10% of patients and twice the rate of placebo) for XEOMIN were eyelid ptosis (19%), dry mouth (16%), visual impairment (12%), diarrhea (8%), and headache (7%).
Drug Interactions
Concomitant treatment of XEOMIN and aminoglycoside antibiotics, spectinomycin, or other agents that interfere with neuromuscular transmission (e.g., tubocurarine-like agents), or muscle relaxants, should be observed closely because the effect of XEOMIN may be potentiated.
Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. XEOMIN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Cost
The costs are expected to be similar to Botox. I checked over on Medical Spa RX and they don't seem to be carrying it as of now so you won't be able get a deal on it the way you can with Rx's Botox Group Buy Program.
Have you got any intention of trying something besides Botox or Dysport? Does Xeomin have a chance in your clinic? Would you try it on a few patients to see if you like it?