Doctors and clinics must adapt to new AI tools for automation, care pathways, and patient engagement to adapt to the new A.I. world - or lose…
In the past few years, the world has seen a massive shift in healthcare. The rise of artificial intelligence and technology has created an opportunity for clinics and doctors to improve patient outcomes and generate recurring revenue through automated care pathways. The need to adapt to these changes is high, with patients already using these technologies and waiting for doctors to catch up. Clinics and doctors who fail to adapt risk becoming irrelevant and losing patients to those who do.
Note: All of the stats and sources used are in the reference section at the bottom of the post.
The world has already shifted to AI.
The statistics speak for themselves. Since 2020, there has been a 63x increase in telemedicine and it’s probably that you’ve already got some kind of video telehealth solution in place, but guess what? It’s already out of date.
71% of all in-person clinical visits can now be done remotely and 89% of all patient interactions are redundant and could be automated. (In fact, the global market for AI in healthcare is expected to grow at a CAGR of 44.9% through 2027, indicating the widespread adoption of these tools.)
Think about what that actually means. Most of what you’re doing in-clinic is essentially redundant and just wasting time and productivity.
Yeah, so the bad news is that you’re already losing money and falling behind.
Worse for you, the top 3 things that patient wants - especially in retail medicine - are 1) more time with caregivers that listen, 2) a feeling that their provider cares personally about their outcome, and 3) more information about their condition, treatment, care, and options. However, your current system of manually reviewing treatment options, recovery, and care prevents you from filling those needs in any type of scalable way. You’re forced by market economics to ration your time, and curtail patient access.
The result is systems that are expensive, highly variable, and slow. In the past the fix has been to hire more people and spend more time at the clinic, but that’s no longer effective.
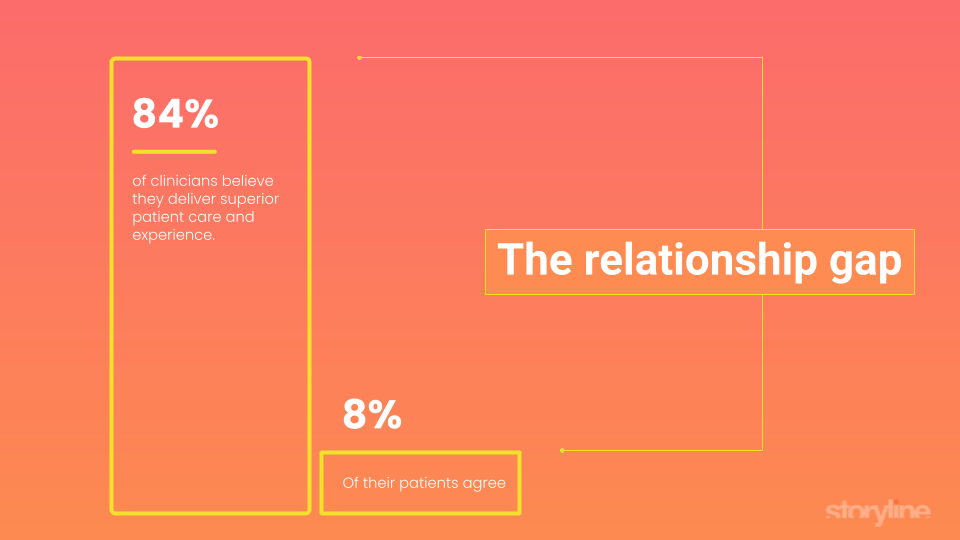
Here’s some numbers that should keep you up at night 62% of patients admit they don’t understand their doctor's advice and the information discussed during a visit. Less than 30% of primary care clinics are financially healthy. 57% of doctors work 70+ hours a week, but 73% of their time is spent not providing care. Clinicians believe they deliver superior patient care and experience, but only 8% of their patients agree. 68% of patients leave because they believe their physician doesn’t care about them, and 81% of existing patients are open to switching providers at any time.
While most clinics feel like they’re adapting, they’re not really. Just because you’re offering telehealth visits or offering check in on an iPad ain’t it. Ditto for appointment reminder emails and the new ‘app’ you just got sold on — they might seem like significant steps forward, but they don't truly address the core issues. These changes are only skin-deep. They don’t fundamentally address the growing patient demand for personalized, efficient, and high-quality care.
There are now 4 million apps in the app store and another 100,000 added every month. No one want’s a one-trick pony
There will be winners and losers. Let’s be a winner.
However, there is a massive new opportunity for first movers who adapt to these changes to grow their practice and their revenue.
While some clinic owners will attempt to keep up with the changes by piecing together multiple disparate systems, this leads to a chaotic, inefficient hodgepodge of tools, each demanding overhead and training and constant attention that force you to struggle with inefficiencies and operational bottlenecks. The winners in this new era of healthcare will be those who embrace integrated care pathways to deliver seamless, personalized patient experiences inside a single, cohesive platform that simplifies operations and workflows.
The right A.I. tools let you do some amazing things that are not immediately obvious, like building in compassion and caring into practice, or measuring their willingness to comply with your treatment plan, or automatically building deep patient relationships that a scale (clinics with strong patient relationships retain an average of 89% of patients as opposed to 33% for clinics with weak engagement).
Best of all, you have a chance to move from a manual fee for service model into a hybrid services + recurring revenue business model (subscriptions) that dramatically scale your business and keep you and working at the top of your license.
Here’s another stat; A 5% reduction in patient churn leads to a significant increase in patient retention, which in turn boosts the lifetime value of each patient. This enhanced lifetime value directly translates to a 25% increase in total profitability, as retaining existing patients is more cost-effective than acquiring new ones and fosters long-term, loyal relationships.
A massive new opportunity for early adopters.
To move beyond the BS and benefit from this new world you need a comprehensive platform.
It’s a long list.
Telemedicine, automated care pathways, user-friendly patient portals, e-signatures, and HIPAA compliance. Additionally, they require tools for patient self-enrollment, automated notifications, mobile accessibility, top-notch security, integrated payments, advanced analytics, and a library of clinically proven resources powered by behavioral A.I. all wrapped up in lightning-fast workflows.
But there’s good news, too.
It’s already being used by the savviest clinics.
Small cosmetic medical clinics now have access to A.I. healthcare tools due to the drop in price and increased affordability of these technologies. The cost of cloud computing has fallen by over 70% in the past five years, making it more accessible to smaller clinics. This has led to an increase in the use of A.I. tools such as image analysis, predictive modeling, and natural language processing to improve patient outcomes.
Fortunately, many of these new AI tools have already been proven in enterprise deployments and are now available for everyone.
Adapting to this new world may seem daunting, but it is necessary for clinics and doctors to thrive in the future. By embracing AI tools and technology, clinics can automate redundant work, build care pathways that improve patient outcomes and engagement, and create a scalable and profitable business model. The opportunity is there for those who are willing to take the leap.
Get a free demo of these new tools:
My recommendation is to get a demo of these new tools and see what they can do for you.
Storyline, which is the only comprehensive platform. It’s also free to use.
I’m not just saying that as the CEO, (Disclaimer: I’m the CEO of Storyline). Other people say that too.
Storyline is trusted by researchers and clinicians at major research universities like Huntsman Mental Health Institute, University of Utah, Moffitt Cancer Institute, Rubicon AI, DepoIQ, Arizona State University, and others.
And now you can use Storyline for free.
Clinics using Storyline have seen a 4x increase in team productivity, a 260% increase in patient interactions without additional provider burden, and an 82% of patient follow-up is automated. Additionally, they have seen a 17% increase in total revenue and Storyline has a 4.9-star patient rating.
Get a demo of Storyline, ask some questions, and kick the tires.
Here’s a big button to get started.